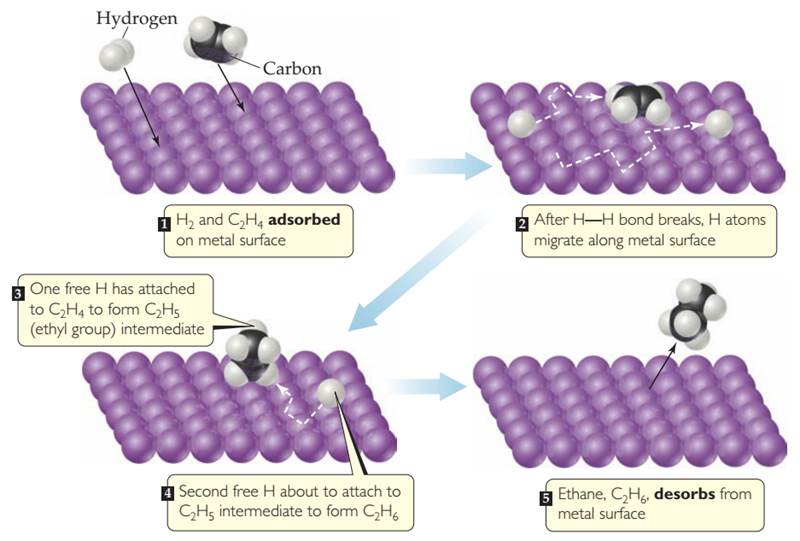
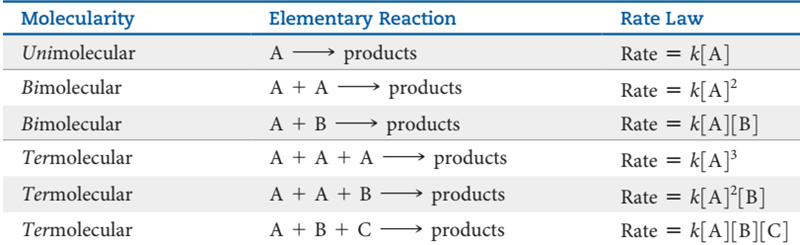
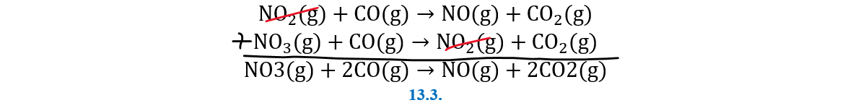| Ciencias de Joseleg
| Química | Química del equillibrio | Cinética
química | (Ejercicios) (Introducción)
(Generalidades)
(Factores
que afectan la velocidad de reacción) (Historia)
(Velocidad
de reacción) (Velocidades
de aparición y desaparición) (Relación
entre velocidades de aparición, desaparición y reacción) (Ley
de velocidad de reacción) (Reacciones
de primer orden) (Reacciones
de segundo orden) (Reacciones
de cero orden) (Vida
media) (Energía
de activación) (Mecanismo
de reacción) (Catalizadores)
(Referencias)
Atkins, P. W., De Paula, J., & Keeler, J. (2018). Atkins’ physical chemistry. Oxford university press.
Baeza-Baeza, J. J., & García-Alvarez-Coque, M. C. (2014).
Extent of Reaction Balances: A Convenient Tool to Study Chemical Equilibria. World
J. Chem. Educ, 2, 54–58. Retrieved from
https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1015.5804&rep=rep1&type=pdf
Brady, J. E., & Humiston, G. E. (1986). General
Chemistry: Principles and Structure. Wiley & Sons.
Brown, T. L., LeMay, H. E. J., Bursten, B. E., Murphy, C. J.,
& Woodward, P. (2009). Chemistry the central science (11th ed.).
Pearson; Prentice Hall.
Brown, T. L., LeMay, H. E. J., Bursten, B. E., Murphy, C. J.,
Woodward, P., & Stoltzfus, M. W. (2015). Chemistry the Central Science.
Chang, R. (2006). Chang’s “General Chemistry - Essential
Concepts” (4th ed.). McGraw-Hill New York.
Chang, R. (2010). Chemistry (10th ed.). McGraw-Hill New
York.
Chang, R., & Overby, J. (2011). General Chemistry,Th e
Essential Concepts (11th ed.). McGraw-Hill New York.
Ebbing, D. D., & Gammon, S. D. (2008). General
chemistry. (Houghton Mifflin Company, Ed.) (9th ed.). Bonston.
Ferner, R. E., & Aronson, J. K. (2016). Cato Guldberg and
Peter Waage, the history of the Law of Mass Action, and its relevance to
clinical pharmacology. British
Journal of Clinical Pharmacology, 81(1),
52–55.
García-García, J. L. (2021). Hacia un equilibrio químico
verdaderamente analítico. Educación Química, 32(1), 133–146.
Retrieved from http://revistas.unam.mx/index.php/req/article/view/75075
Garst, J. F. (1974). The extent of reaction as a unifying
basis for stoichiometry in elementary chemistry. Journal of Chemical
Education, 51(3), 194. Retrieved from
https://files.eric.ed.gov/fulltext/EJ1179603.pdf
Gilbert, T. R., Kirss, R. V, Foster, N., & Davies, G.
(2012). Chemistry, the science in context (3rd ed.). W. W. Norton &
Company, Inc.
Gorzynski, J. (2010). General, organic, and biological
chemistry (1st ed.). McGraw-Hill Education.
Hanyak Jr, M. E. (2014). Extent of Reaction or Events of
Reaction?
IUPAC. (2020). Compendium of chemical terminology.
(International Union of Pure and Applied Chemistry, Ed.) (Version 3.).
Blackwell Science Oxford. Retrieved from https://goldbook.iupac.org/
IUPAC, McNaught, A. D., & Wilkinson, A. (2019). extent of
reaction, ξ. In Compendium of Chemical Terminology, 2nd ed. (the “Gold
Book”) (2nd ed.). Blackwell Scientific Publications, Oxford. Retrieved from
https://goldbook.iupac.org/terms/view/E02283
Jespersen, N. D., Brady, J. E., & Hyslop, A. (2012). Chemistry
The Molecular Nature of Matter (6th ed.). USA: Wiley.
Matamála, M., & Gonzalez, P. (1976). Química General.
Cultural.
Moretti, G. (2015). The
“extent of reaction”: a powerful concept to study chemical transformations at
the first-year general chemistry courses. Foundations of Chemistry, 17(2),
107–115.
Petrucci, R. H., Harwood, W. S., & Herring, F. G. (2003). Química
General (8th ed.). Prentice Hall.
Petrucci, R. H., Herring, F. G., Madura, J. D., &
Bissonnette, C. (2010). General Chemistry Principles and Modern Applications
(10th ed.). Pearson.
Ptáček, P., Opravil, T., & Šoukal, F. (2018). A Brief
Introduction to the History of Chemical Kinetics. Introducing the Effective
Mass of Activated Complex and the Discussion on the Wave Function of This
Instanton.
Raymond, K. W. (2014). General, Organic, and Biological Chemistry,
an Integrated Aproach (4th ed.). Wiley.
Silberberg, M. S. (2009). Chemistry, The Molecular Nature
of Matter and Change (5th ed.). McGraw-Hill Education.
Timberlake, K. C. (2015). Chemistry An Introduction to
General, Organic, and Biological Chemistry (15th ed.). USA: Pearson.
Whitten, K. W., Davis, R. E., Peck, M. L., & Stanley, G.
G. (2010). Chemistry (9th ed.). Brooks/Cole.
Zumdahl, S. S., & Zumdahl, S. A. (2007). Chemistry
(7th ed.). Boston: Houghton Mifflin Company.
Zumdahl, S. S., Zumdahl, S. A., & DeCoste, D. J. (2018). Chemistry
(10th ed.). Cengage Learning.